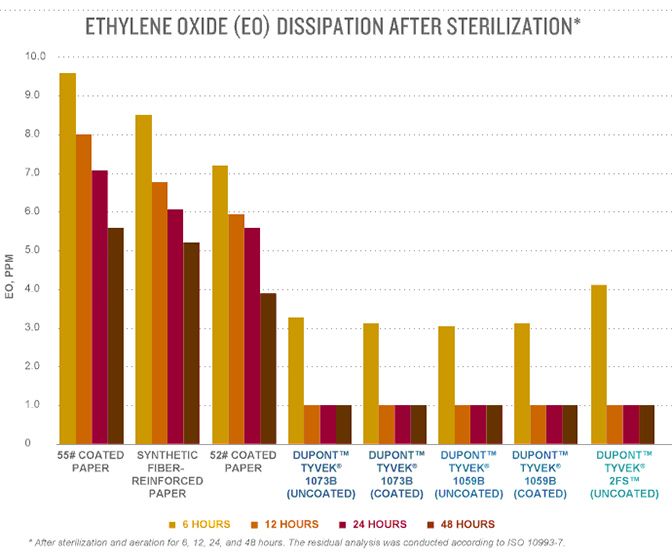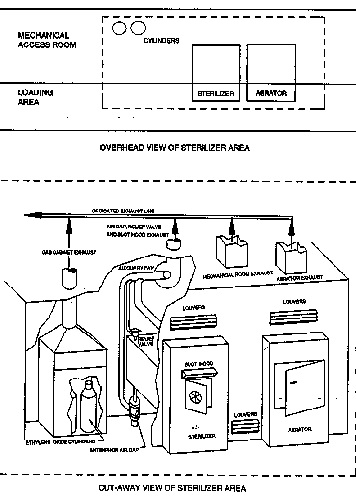
Biocompatibility & analysis of medical devices according to ISO 10993 - ISO 10993-7: Ethylene oxide sterilization residuals - Services - Danish Technological Institute
UNE EN ISO 10993-7:2009 Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals (ISO 10993-7:2008) - European Standards

Identification of residues in ethylene oxide sterilized hard gelatin capsule shells by gas chromatography-mass spectrometry and development of a simple gas chromatography-flame ionization detector method for the determination of residues - ScienceDirect

Impact of Ethylene Oxide Sterilization of Polymer-Based Prefilled Syringes on Chemical Degradation of a Model Therapeutic Protein During Storage - Journal of Pharmaceutical Sciences

Thoughts on amendments to ISO 10993-7 medical device ethylene oxide sterilization residuals - Pharmaceutical Technology

Effect of Ethylene Oxide and Gamma (γ-) Sterilization on the Properties of a PLCL Polymer Material in Balloon Implants | ACS Omega

AAMI TIR19:1998 -- Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals: AAMI: Amazon.com: Books