Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

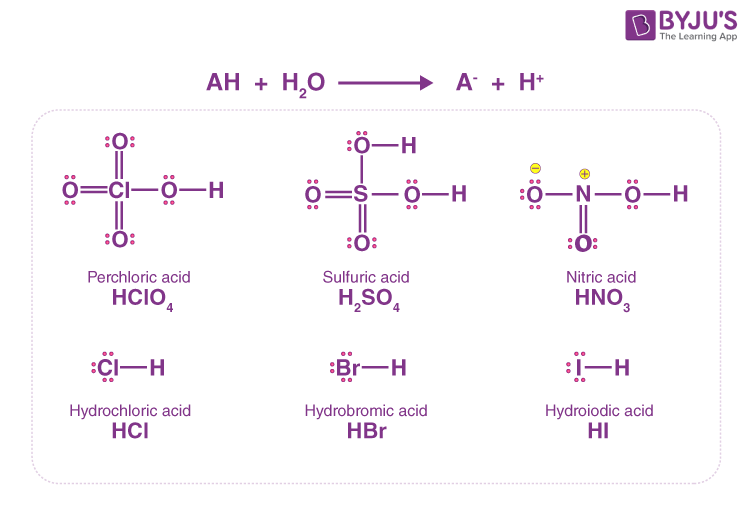
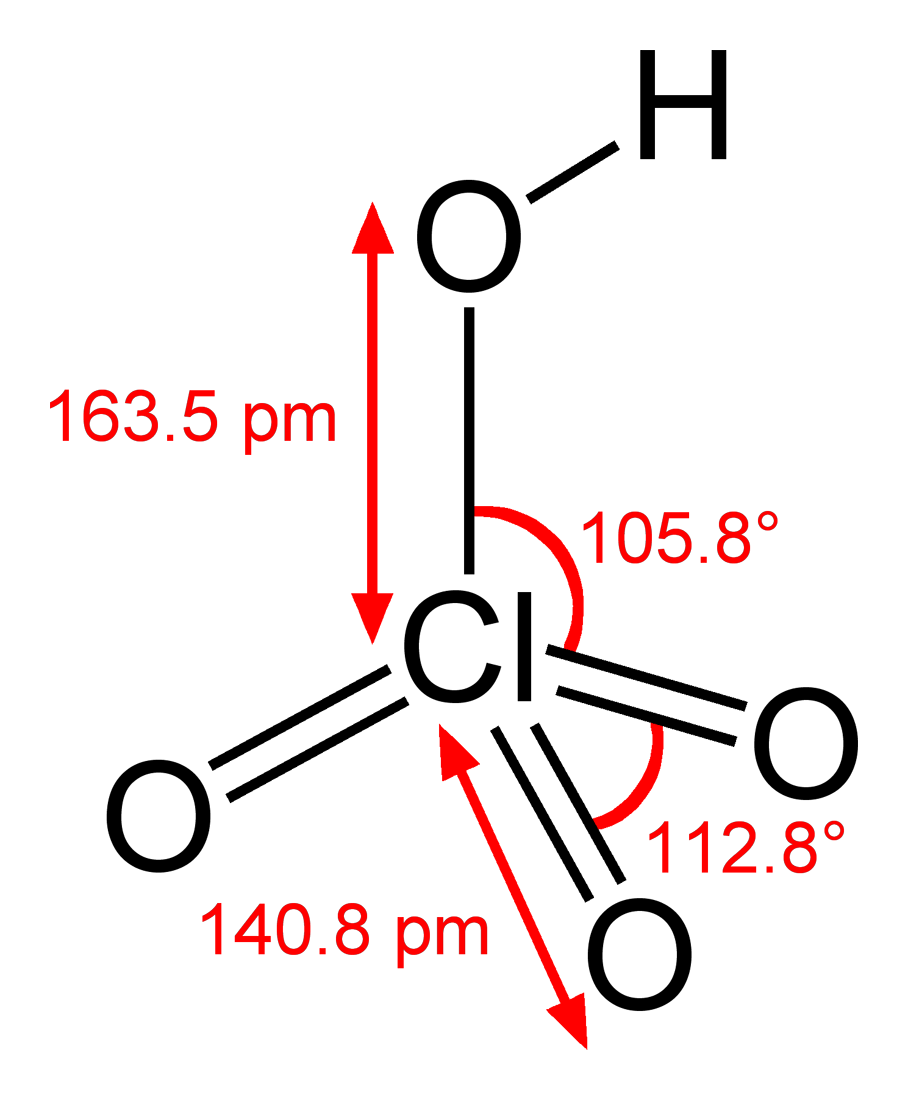
Difference Between Perchloric Acid and Hydrochloric Acid | Compare the Difference Between Similar Terms

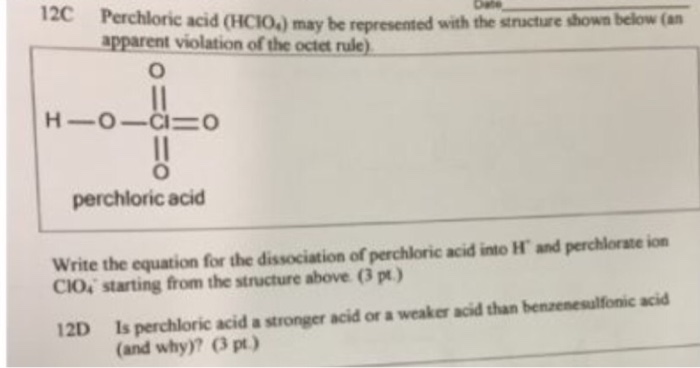
Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

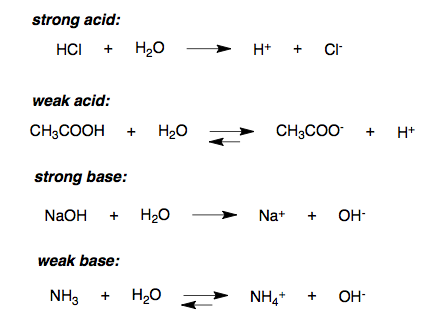
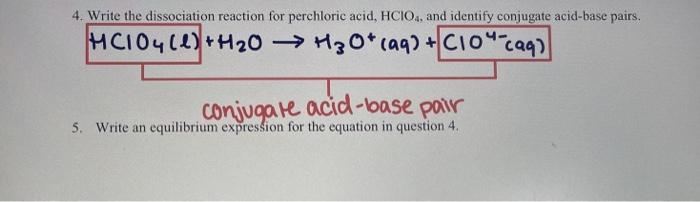
Strong Acids Ions are present in an aqueous solution of an acid, because these ions result from the dissociation of the acid. An acid that dissociates. - ppt download
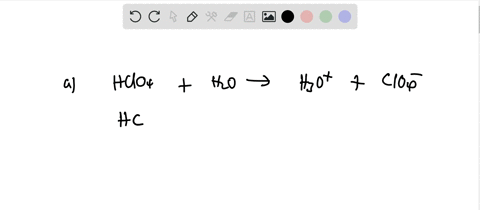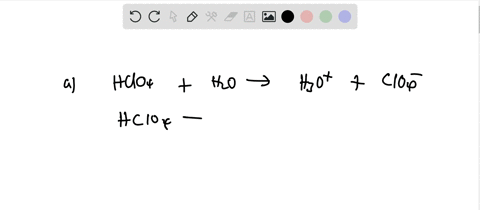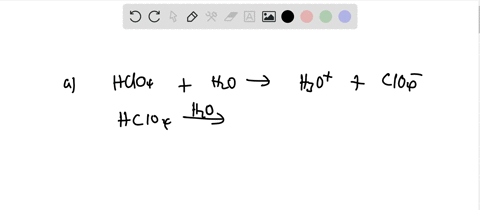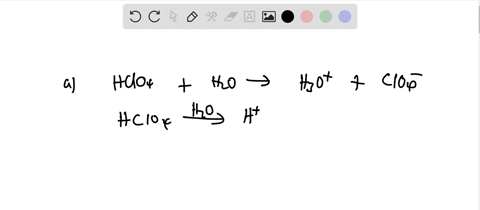
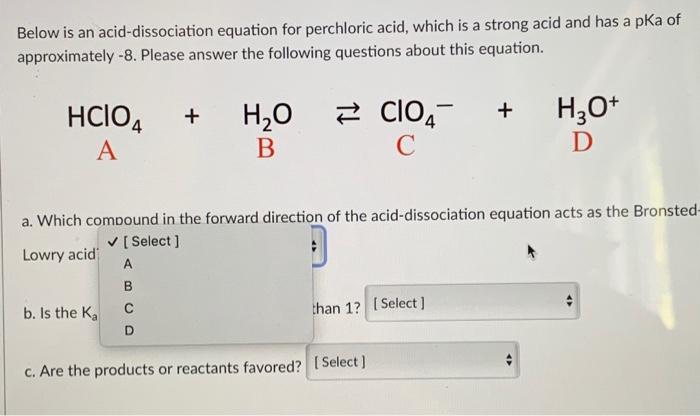
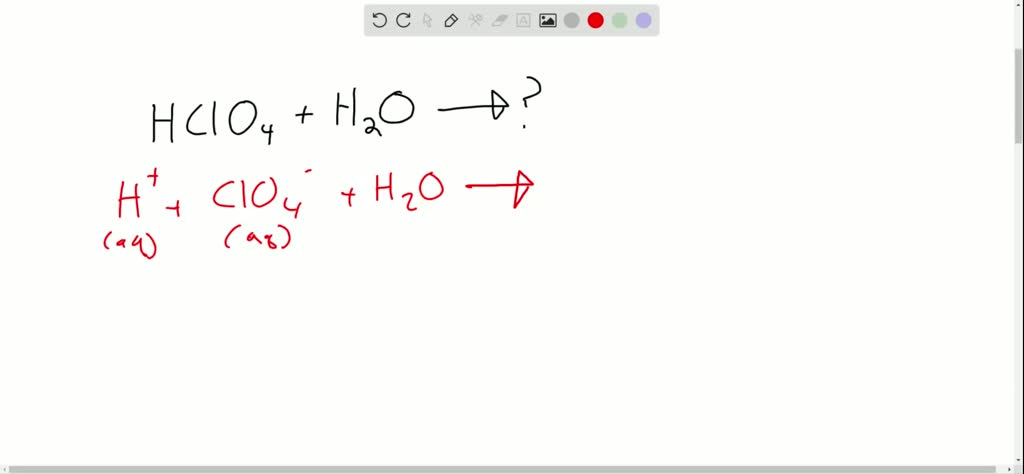
SOLVED: Perchloric acid, HClO4, is a strong acid. (a) Write the chemical equation for the reaction between perchloric acid and water. (b) List all species present in an aqueous solution of perchloric

Representative in vitro 31 P-NMR spectra (perchloric acid extracts,... | Download Scientific Diagram