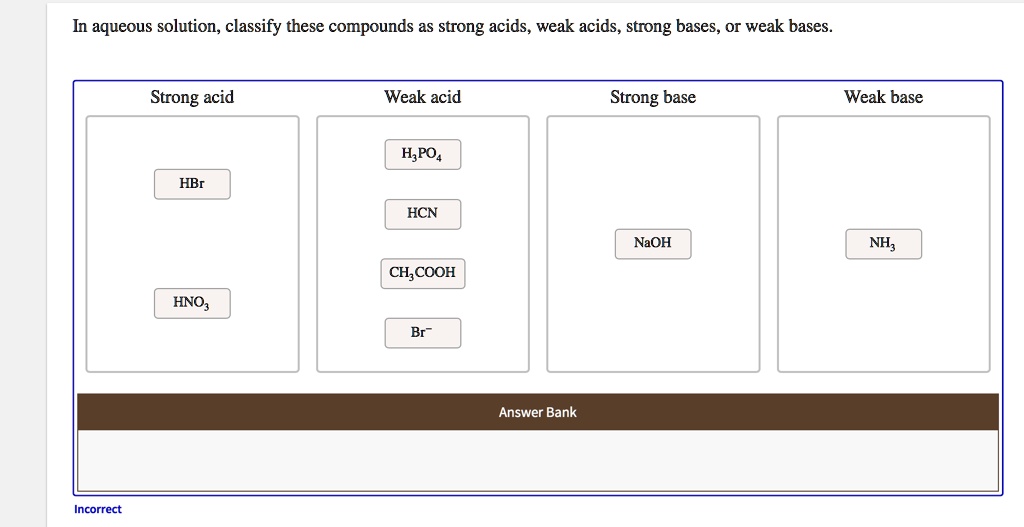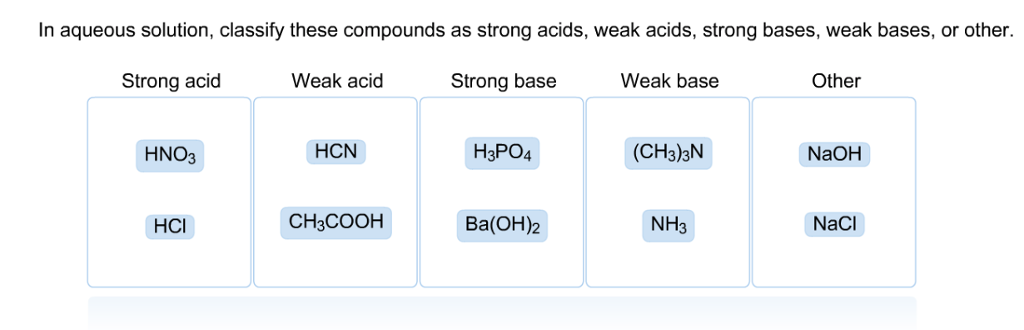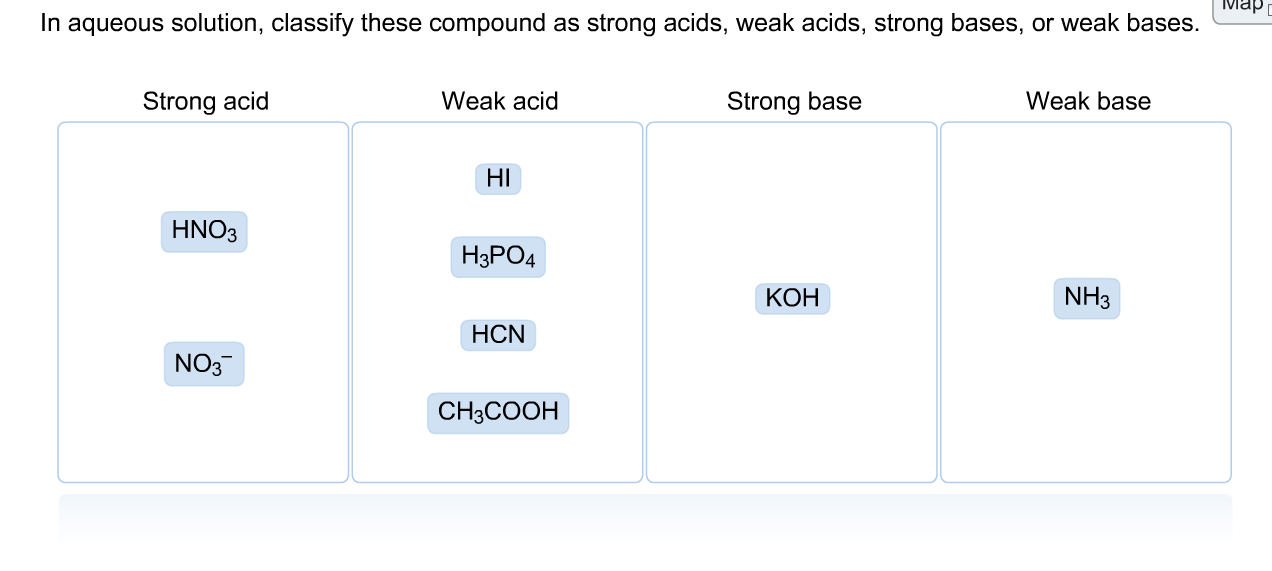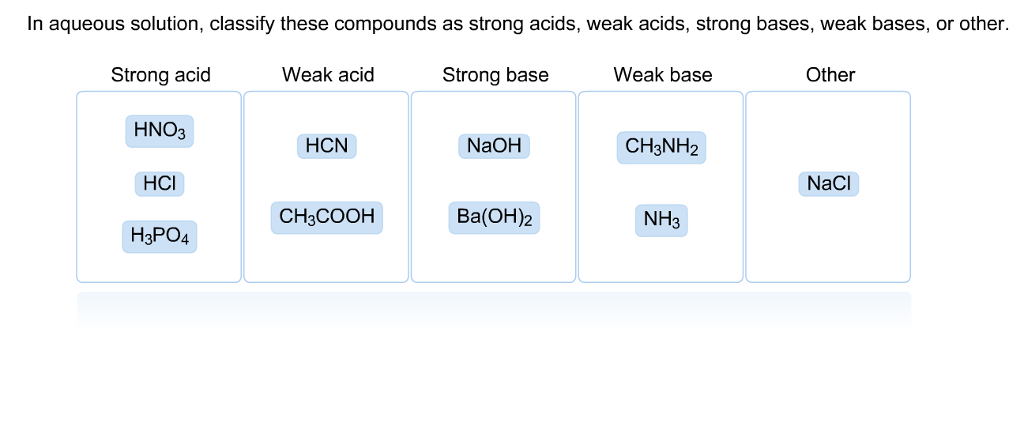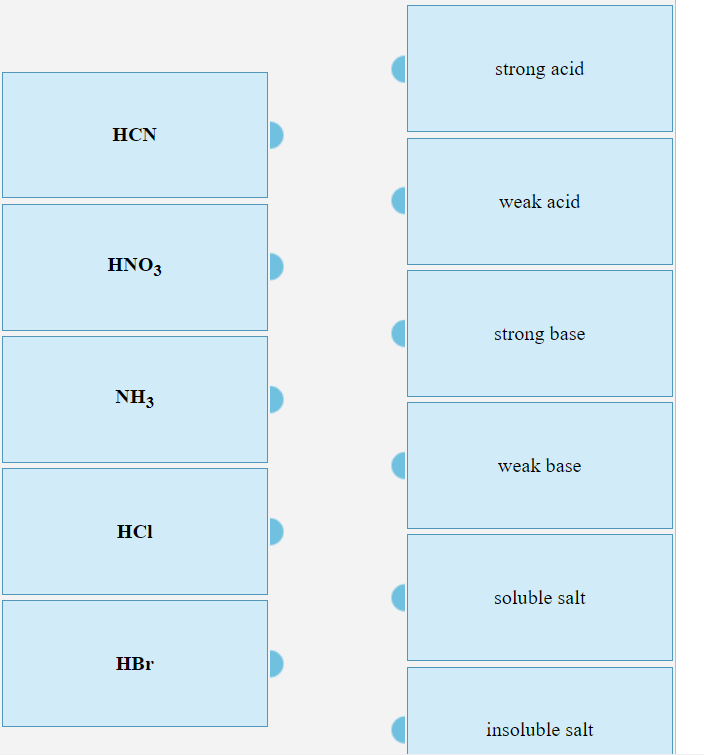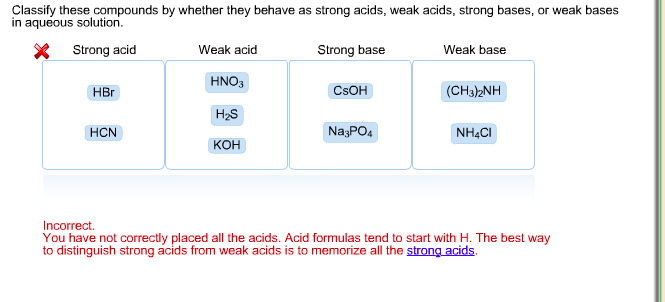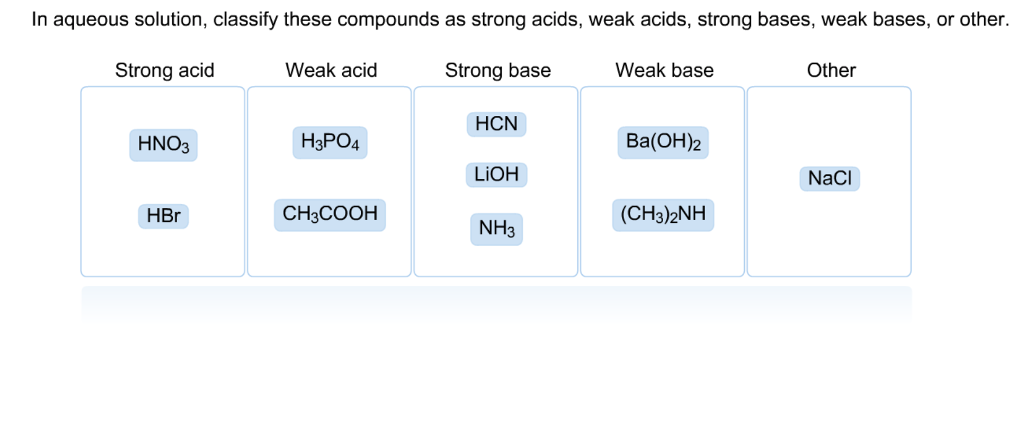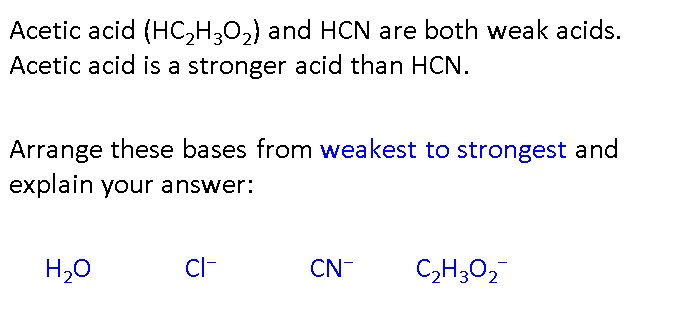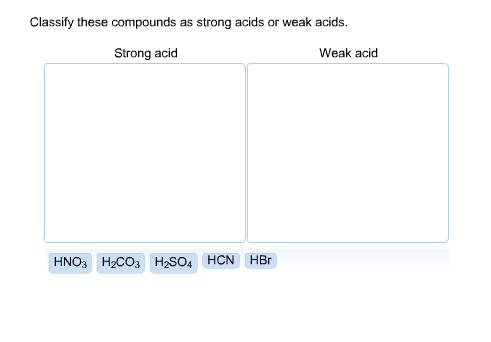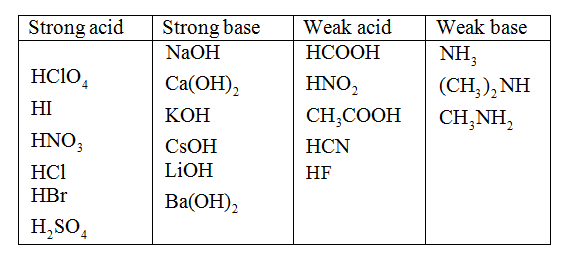
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum
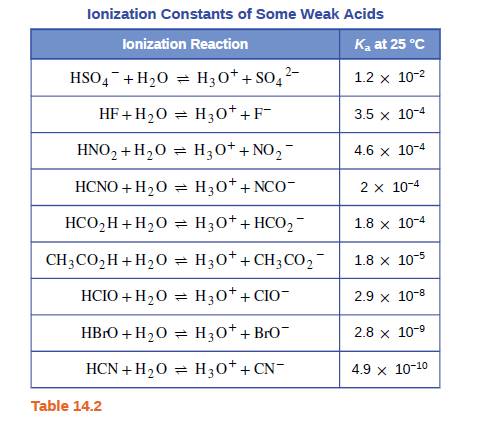
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases. F“ or CN”, is the stronger base? See Table 14.2. | bartleby

Which should be stronger acid, HOCN, or HCN? Explain briefly In HOCN, the H+ ion is attached to th - YouTube

The heat of neutralization of a strong base and a strong acid is 13.7 kcal. The heat released when 0.6 mole HCl solution is added to 0.25 mole of NaOH is:

HCN is a weak acid ( Ka = 6.2 × 10^-10 ) ,NH4OH is a weak base ( Kb = 1.8 × 10^-5 ) . A 1.00 M solution of NH4CN would be: