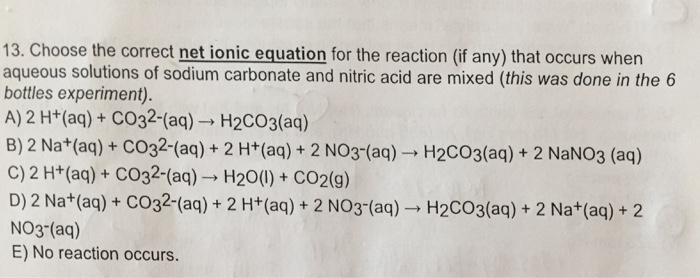Effect of sodium carbonate, nitric acid, and acidic mixture on Akunsa... | Download Scientific Diagram
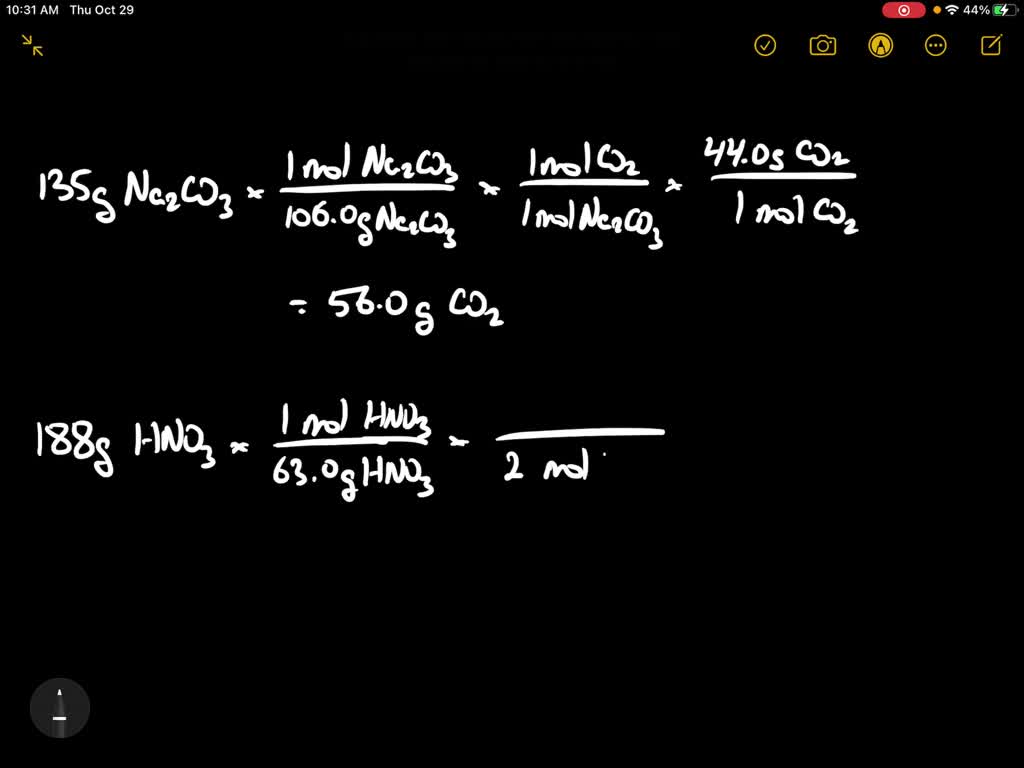
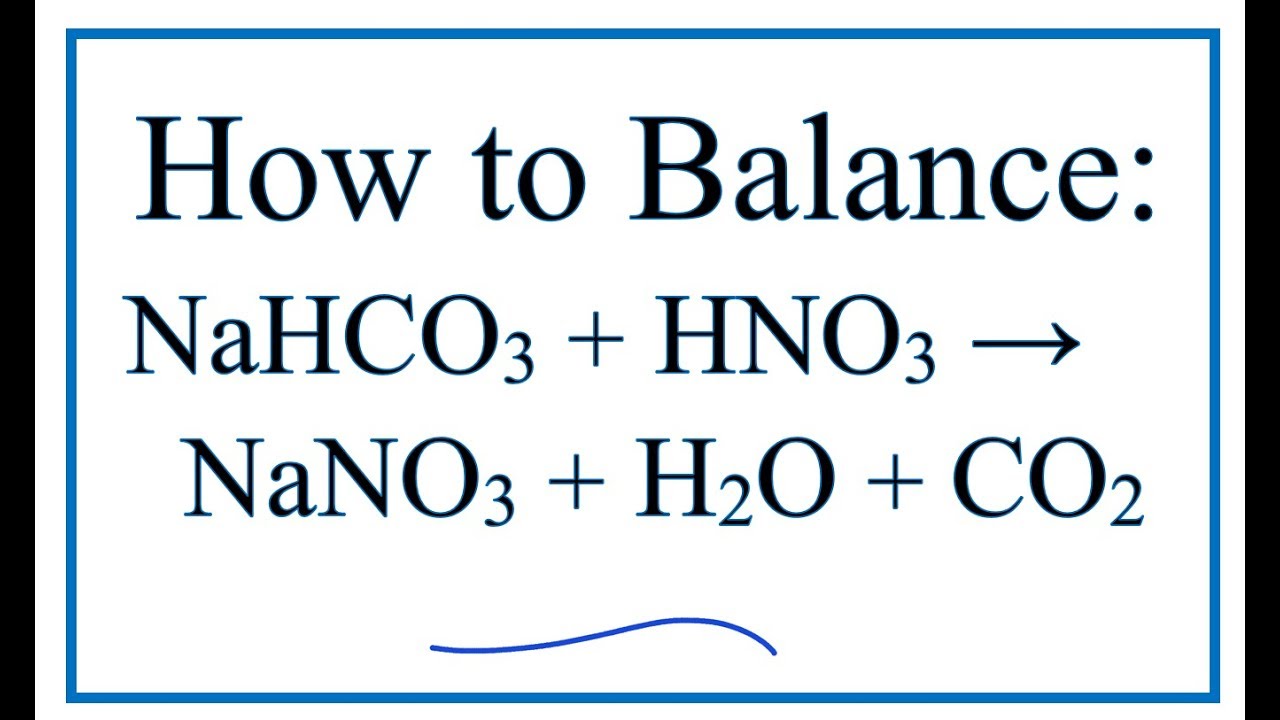
SOLVED:Sodium carbonate can neutralize nitric acid by the reaction 2 HNO3+Na2 CO3 →2 NaNO3+H2 O+CO2 . Is 135 grams of sodium carbonate enough to neutralize a solution that contains 188 grams of
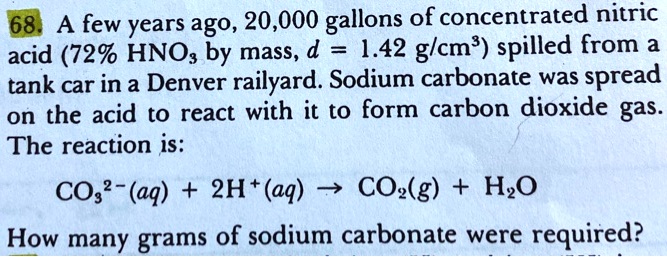
SOLVED: 68. A few years ago, 20,000 gallons of concentrated nitric acid (729 HNOs by mass, d 1.42 glcm?) spilled from tank car in a Denver railyard. Sodium carbonate was spread on

SOLVED: Question 7 (1 point) Sodium carbonate reacts with nitric acid to form sodium nitrate; carbon dioxide, and water according to the balanced reaction below How many grams of sodium carbonate are

Sodium carbonate salt chemical structure, illustration - Stock Image - F027/9408 - Science Photo Library

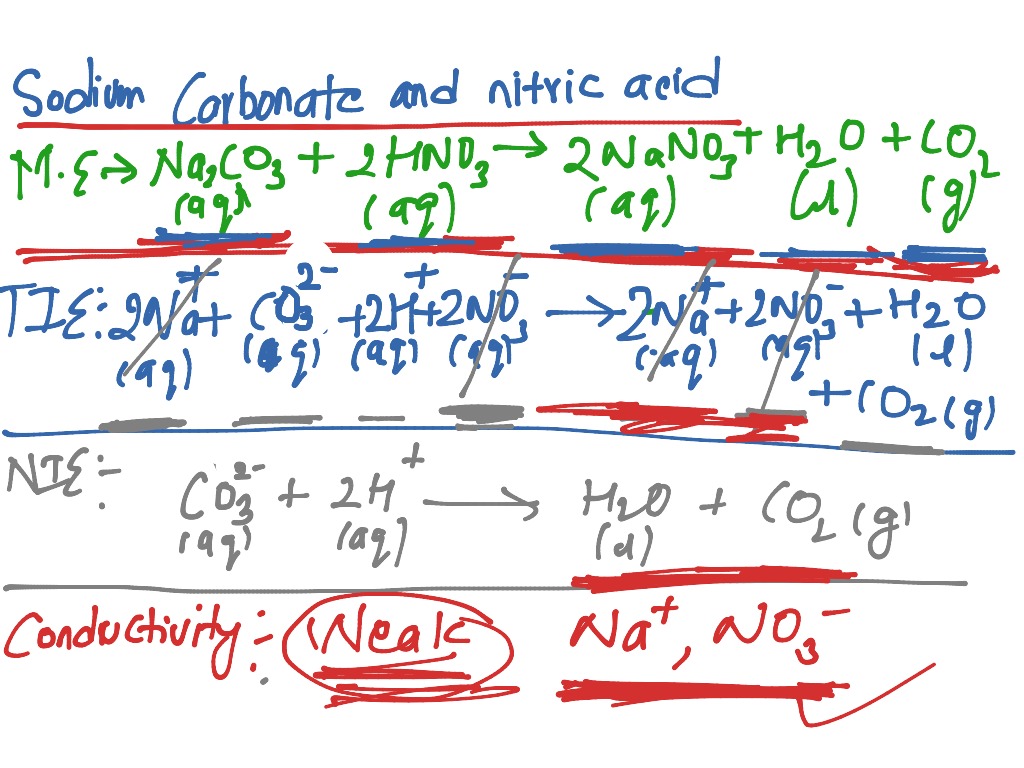
OneClass: Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and so...


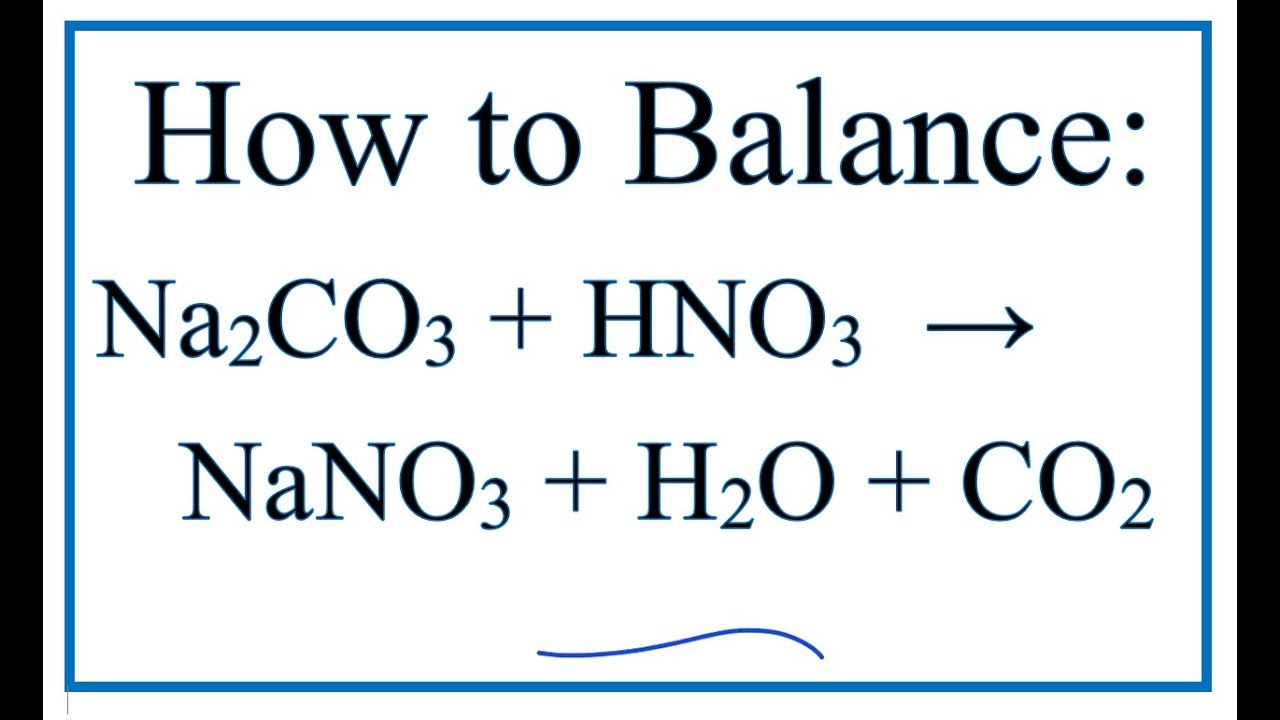

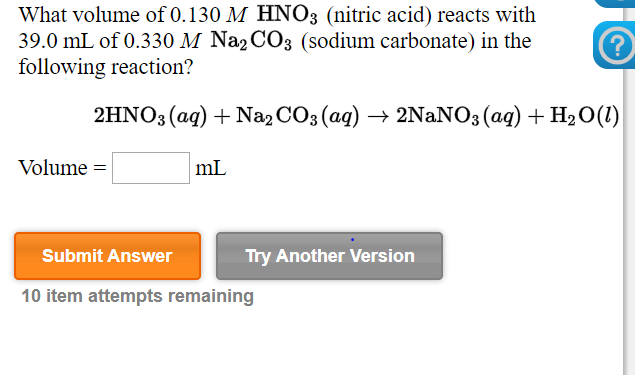

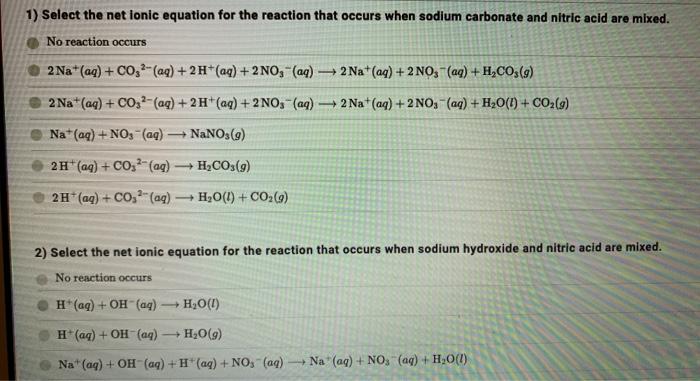
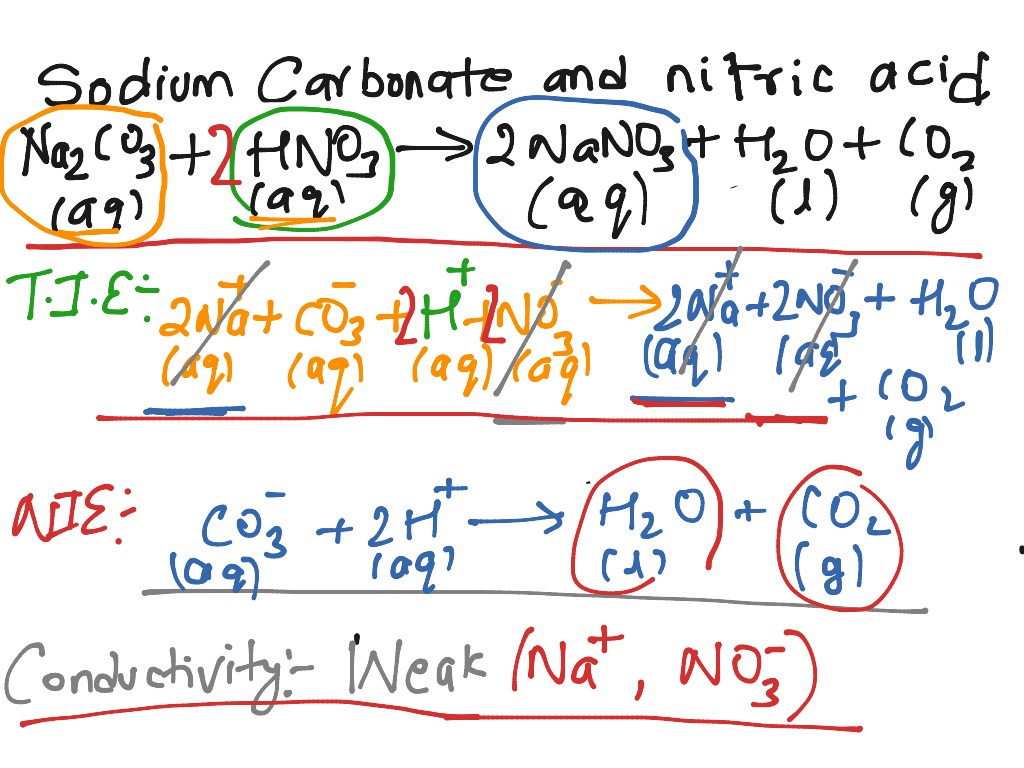
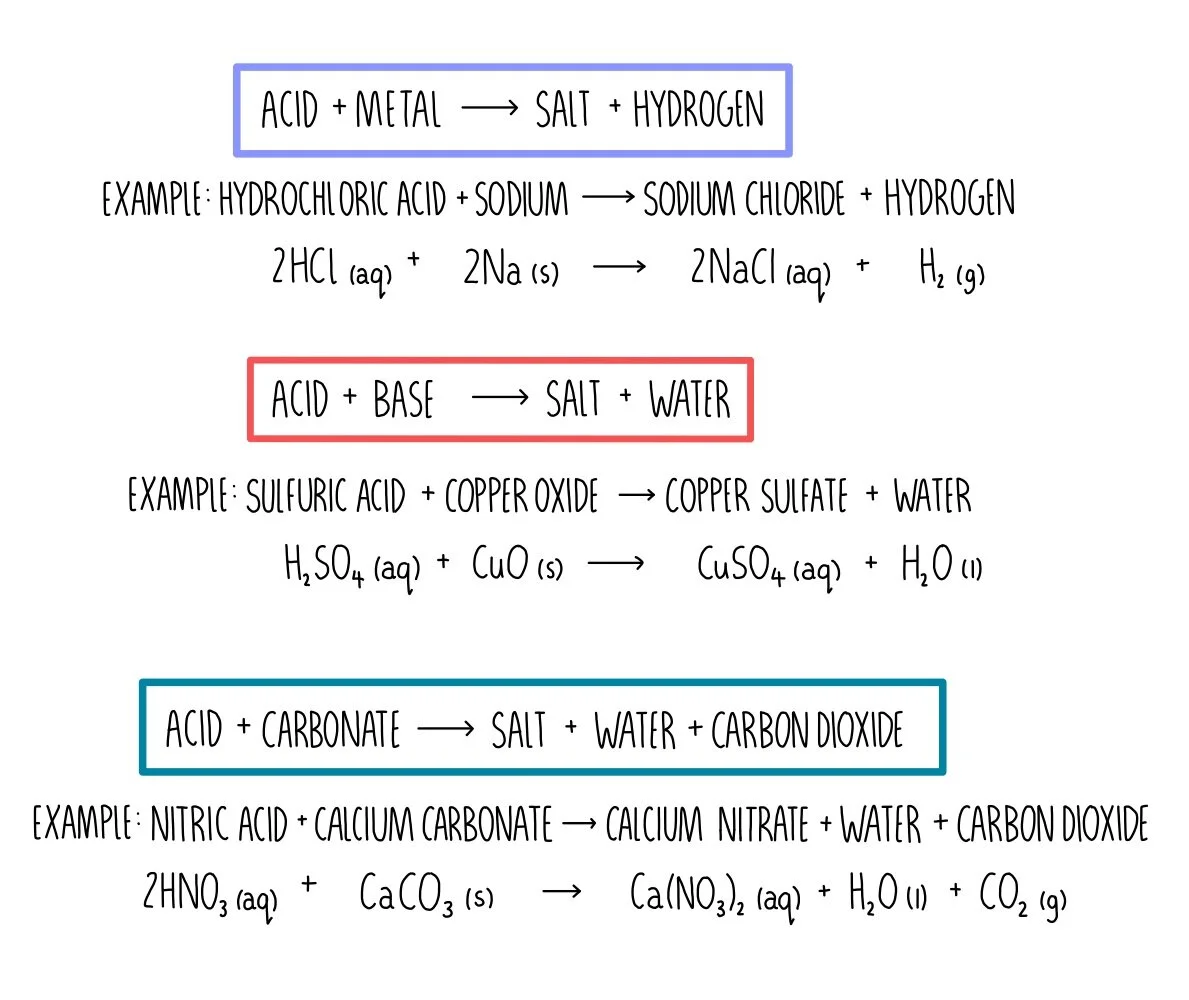
![ANSWERED] When nitric acid (HNO3) reacts with sodium... - Organic Chemistry ANSWERED] When nitric acid (HNO3) reacts with sodium... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/59811332-1659634030.9246697.jpeg)