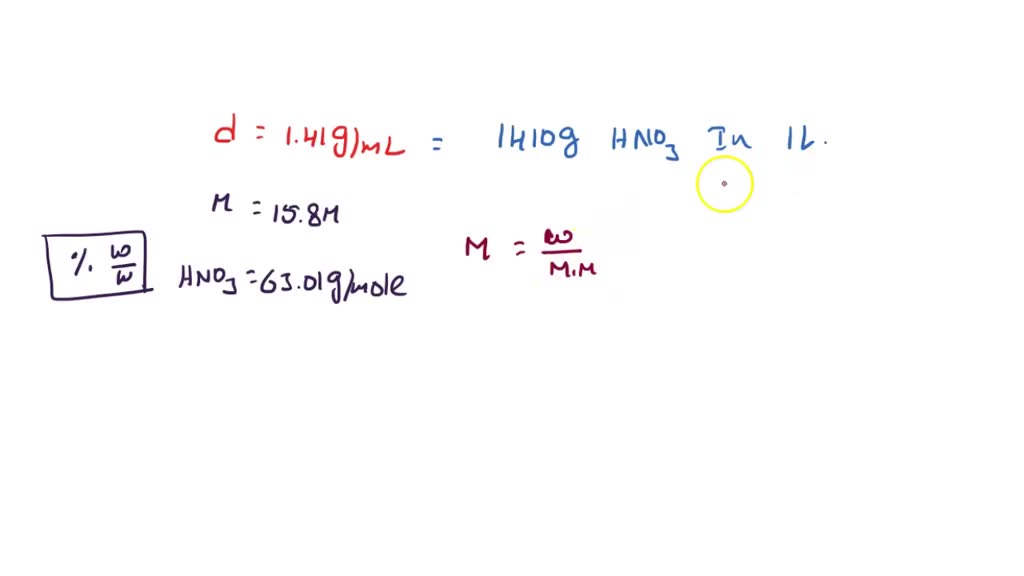
SOLVED: A concentrated nitric acid solution has a density of 1.41 g/mL at 25 C and is 15.8 M. What is the percent by mass of HNO3 in the solution? a. 70.6 %

Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. - YouTube

SOLVED: A bottle of concentrated aqueous nitric acid labeled 65% HNOz has density 1.4 g/ml (Mwt =63.01 g/mol) 1What is the molarity of this solution? ILII 214.44 1222 2How many mls of
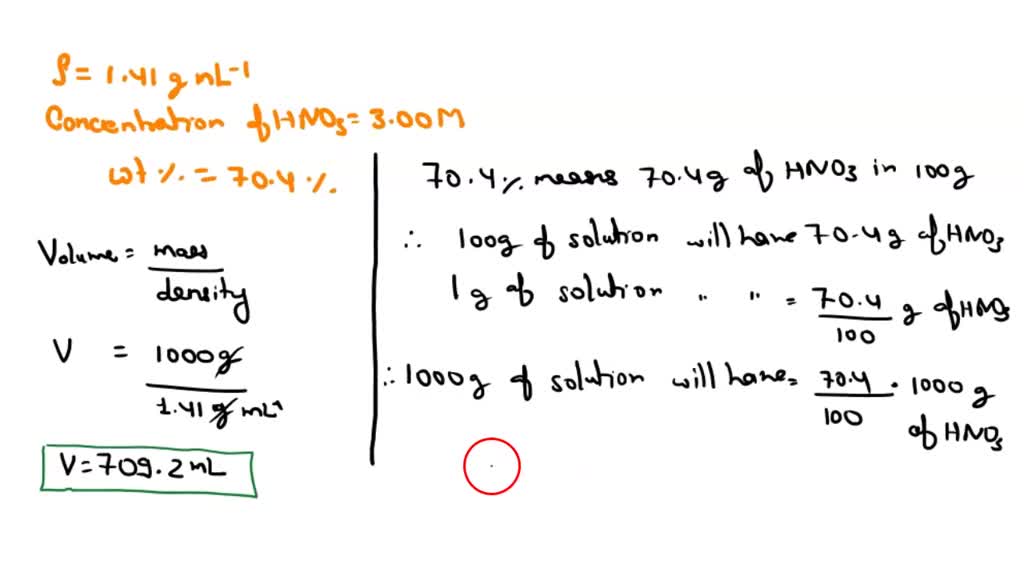
SOLVED: Calculate how many mL of 70.4 wt% nitric acid should be diluted to 0.250 L to make 3.00 M HNO3 (density of nitric acid is 1.41 g/mL)
Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. - Sarthaks eConnect | Largest Online Education Community

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube
Calculate the concentration of nitric acid in miles per litre ina sample which has a density 1.41g/mL and the mass percent of nitric acid in it being 69percent.

Calculate the concentration of nitric acid in moles per litre in a sample which has a density.... - YouTube

Concentrated nitric acid used in the laboratory work is `68%` nitric acid by mass in aqueous sol... - YouTube

Concentrated `HNO_(3)` is 69% by mass of nitric acid. Calculate the volume of the solution which - YouTube

![PDF] Nitric Acid, Nitrous Acid, and Nitrogen Oxides | Semantic Scholar PDF] Nitric Acid, Nitrous Acid, and Nitrogen Oxides | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/9dca15c058c7c8f03a631c47da507d6f57448151/3-Table1-1.png)

![Solved Question 2 [24 Marks] 2.1 A nitric acid solution in | Chegg.com Solved Question 2 [24 Marks] 2.1 A nitric acid solution in | Chegg.com](https://media.cheggcdn.com/study/221/2218afbb-3f5f-4ab6-a770-8fd2a6094b4d/image.png)